First-Ever Nasal Spray for Life-Threatening Allergic Reactions Approved
The epinephrine nasal spray Neffy, a needle-free alternative to the EpiPen, is approved to treat emergency anaphylaxis. Here's the latest.

The US Food and Drug Administration (FDA) has approved the first ever life-saving nasal spray for the treatment of severe allergic reactions. The approval, announced Friday, offers an alternative to the millions of people who until now have had to rely on injections—known to most as EpiPens. The nasal spray, which will be sold under the brand name Neffy, is designed to be used in place of auto-injectable pens filled with epinephrine (aka adrenaline, a hormone that can be used in drug form to treat allergic emergencies). The new drug only requires a single spray into one nostril and is approved for use in adults and children weighing over 66 pounds.
“Today’s approval provides the first epinephrine product for the treatment of anaphylaxis that is not administered by injection,” Kelly Stone, MD, PhD, associate director of the Division of Pulmonology, Allergy and Critical Care at the FDA, said in a statement. “Anaphylaxis is life-threatening and some people, particularly children, may delay or avoid treatment due to fear of injections.”
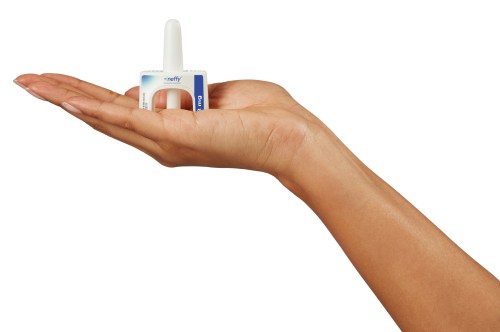
Anaphylaxis—the life-threatening condition that can occur within seconds of encountering an allergen—may occur in one in 50 Americans, according to the Asthma and Allergy Foundation of America. Stats are hard to come by, however, and some believe the number could be as high as one in 20 Americans. “The availability of epinephrine nasal spray may reduce barriers to rapid treatment of anaphylaxis. As a result, neffy provides an important treatment option and addresses an unmet need,” Dr. Stone said in the statement.
In September 2023, the FDA declined to approve the drug and requested additional testing, despite recommendations from independent experts, according to Reuters. Neffy has been tested in 175 healthy adults without anaphylaxis, due to ethical concerns. The concentration of epinephrine in the blood after administration was comparable to those who received an EpiPen injection instead. Neither the FDA, nor the makers of Neffy, ARS Pharmaceuticals, have announced when the drug will be available for purchase.
This comes as welcome news to anyone who personally deals with life-threatening allergies—or knows or cares for someone who does. And, thanks to its new method of delivery, Neffy may prove less intimidating to use at various life and age stages than traditional needle-injections pens.
Sign Up for Our Daily Newsletter
Get all the latest in wellness, trends, food, fitness, beauty, and more delivered right to your inbox.
Got it, you've been added to our email list.










